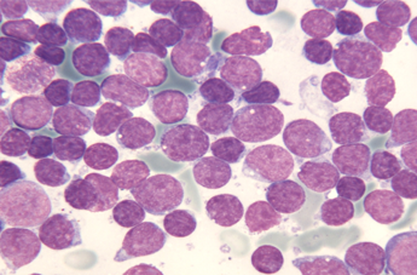
Dr. Josep Mª Ribera is a haematologist, specialised in acute lymphoblastic leukaemia in adults (LLA) and head of the Haematology Clinical Unit in the Catalan Oncology Institute (ICO), within the Trias i Pujol Hospital in Badalona (Barcelona). Very recently, he has taken part as a researcher for the ICO and the Josep Carreras Leukaemia Institute in a worldwide clinical trial (phase II) for the evaluation of a treatment with a new drug, Blinatumomab*, in acute Precursor B-cell lymphoblastic leukaemia patients in relapse. Thanks to the results of this study, recently published in Lancet Oncology, in which 189 ill adults from different international centres have taken part, the American Drug agency (FDA) approved its standard use a few weeks ago.
The patients who can be subject to this treatment are adults diagnosed with acute lymphoblastic leukaemia (LAL) either refractory (resistant to conventional treatment) or in relapse. The administration of Blinatumomab, a bispecific monoclonal antibody, has shown a complete response rate in 43% of the patients.
Currently a phase III study is taking place, which compares Blinatumomab to the best rescue chemotherapy these patients are receiving today, in order to achieve the definitive approval of Blinatumomab in refractory or relapsing LAL patients. The Josep Carreras Leukaemia Institute and the Catalan Oncology Institute in Badalona are also taking part in this trial.
Soon, Blinatumomab will be subjected to the approval of the European Medicines Agency (EMA).
* +info: Blinatumomab is a bispecific monoclonal antibody, anti-CD3 and anti-CD19, which is attached to the leukemic cell (CD19 positive) and to the T cytotoxic (CD3 positive) lymphocyte, so the T lymphocyte kills the leukemic cell directly. This proves the high efficiency of immunotherapy in acute lymphoblastic leukaemia, just like in other haematologic neoplasms.