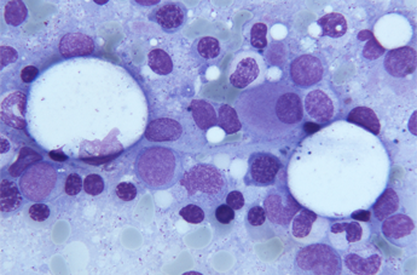
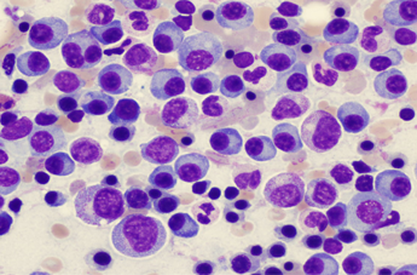
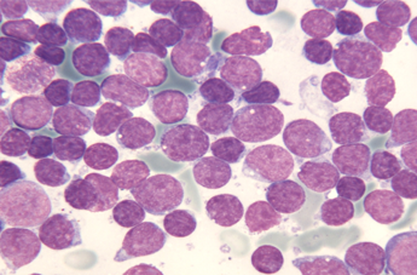
Pablo Menéndez has been awarded a European Research Council Consolidator Grant for his project Genomic, Cellular and Developmental Reconstruction of Infant MLL-AF4+ Acute Lymphoblastic Leukaemia.
The myelodysplastic syndromes are a perfect example for the need for personalised treatment: they are extremely heterogeneous and no single type of treatment is effective in all patients
“It was an excellent idea to build up a special center dedicated to leukaemia and related disorders here in Barcelona. This center is collaborating with international research partners. The exchange to knowledge, techniques and ideas and the development of study protocols and projects with cooperating partners has tremendous impact on scientific progress”.
New indicators for myelodysplastic syndrome prognosis
Dr Francesc Solé, in charge of the myelodysplastic syndrome (MDS) line at the Josep Carreras Research Institute has taken part in an international project recently published in Leukemia magazine, Nature’s leading publication in malignant haemopaties.
The study”Validation of WHO classification-based prognostic Scoring System (WPSS) for myelodysplastic Syndrome and comparison with the revised International prognostic Scoring System (IPSS-R). A study of the International Working Group for Prognosis in Myelodysplasia (IWG-PM)“, establishes a new worldwide benchmark indicator to improve the prognostic stratification of patients suffering myelodysplastic syndromes (MDS).
Since 2012, an international prognosis indicator called IPSS-R (International Prognostic Scoring System) has existed for MD syndromes (Greenberg et al., Blood 2012) but it did not take into account an important indicator to establish a prediction of the development of the disease: transfusional needs. Patients with an MDS diagnosis who require more transfusions have a worse prognosis that can affect their survival or the risk of their disease becoming acute leukaemia. For this reason, since then, work has been done at international level gathering clinical information from as many patients over 16 years old suffering from de novo MDS as possible. By studying this data, researchers all over the world, including Dr Solé’s team, have established a new prognostic classification called WPSS-R, (Della Porta M et al. Leukemia, 2015), which completes the previous one and allows improvement of MDS patient categorization depending on their cytogenetic alterations, the WHO diagnosis, transfusional needs and parameters such as blasts, cytopenia, haemoglobin, platelets and the patients age.
This new clinical indicator will be of great use in low risk myelodysplastic syndromes, for these are the ones that usually have transfusional needs.
New groups for basic research in the Campus ICO-Germans Trias i Pujol Research of the Josep Carreras Leukaemia Research Institute
New groups for basic research in the Campus ICO-Germans Trias i Pujol Research of the Josep Carreras Leukaemia Research Institute
New Tax Reform
Information on the recent tax modifications that are now more beneficial for charitable people
INDIVIDUALS
The first 150 euros donated from January 2015 will have a 50% deduction on their income tax quota (on the 2015 tax return) and a 75% deduction (from the 2016 tax reform onwards).
Also, any amount over 150 euros has an additional 27,5% deduction (on the 2015 tax return) and a 30% deduction (on the 2016 tax return) on top of the one corresponding to the first 150 euros.
Fidelity as a donor will be rewarded with additional tax relief. These last percentages can be increased to 32,5% (2015 tax return) and 35% (2016 tax return), respectively, if in the two previous years donations had been made to the same NGO for an equal or greater amount.
LEGAL ENTITIES
The deduction on the corporation tax quota will be 37,5% (in the 2015 tax return period) and 40% (from 2016 onwards), respectively, if in the two previous tax periods, donations have been made with deduction rights to the same NGO for an equal or greater amount. In other cases, the deduction will be 35%.
If you have any doubts or questions you can get in touch with the Members and Donations Department of the Josep Carreras Foundation through email at amics@fcarreras.es or by telephone at 93 414 55 66.
New progress in the treatment of multiple myeloma patients in relapse
The Josep Carreras Leukaemia Research Institute, together with the Catalan Oncology Institute (ICO) in Badalona, takes part in a new study to improve survival in multiple myeloma patients who don’t respond to regular treatments.
Dr. Albert Oriol, ICO haematologist and researcher for the Josep Carreras Institute, is the researcher who is taking part in this international clinical trial, recently published in the New England Journal of Medicine medical journal. In it, 792 multiple myeloma patients in relapse have been included. A third drug has been added (carfilzomib) to the combination (lenalidomide and dexamethasone) they usually receive.
This variation prolonged the time of response to the treatment before the following relapse by more than half a year (from 18 to 26 months). Just as important as this is the fact that the combination of the three drugs was not more toxic than the two drugs previously administered. Other studies underway are exploring similar combinations, combining more active and powerful drugs with which improved efficiency is not counteracted by higher toxicity.
Towards the end of 2014, Dr. Oriol also took part in another successful international clinical trial through the Josep Carreras Research Institute and the Catalan Oncology Institute. This first study was aimed at the improvement of the treatment for multiple myeloma patients who cannot receive a bone marrow transplant due to their advanced age, which is most of them. View more information HERE.
Multiple myeloma
The multiple myeloma (MM) is a type of blood cancer that affects plasmatic cells, a type of white cell mostly found in the bone marrow. It usually affects elderly people, the average age being 65. Only 15% of patients are under 50 and just 2% are less than 40 years old.
If they require treatment, for patients under 70 it will be based on classic intravenous chemotherapy, which might be joined to one or more of the new agents and followed by an autologous transplant (which has become part of the standard treatment for this disease). The objective of this treatment is always to slow down the development of the disease and improve the symptoms. Regretfully, none of them cures it.
Multiple myeloma is one of the haematologic diseases in which there has been more progress in the last few years. Optimization of the initial treatment has allowed the time to the following relapse to double and the survival of the patients, although most of them eventually relapse and have to be treated again. Traditionally, second-line and further treatments consist in the use of sequential drugs to obtain shorter and shorter responses with the least possible toxicity. Lately, more active combinations are being researched that allow results in relapsing patients to improve too.
The Josep Carreras Leukaemia Research Institute, together with the Catalan Oncology Institute takes part in a study that has lead to the approval of a new drug as a rescue treatment for acute lymphoblastic leukaemia
Dr. Josep Mª Ribera is a haematologist, specialised in acute lymphoblastic leukaemia in adults (LLA) and head of the Haematology Clinical Unit in the Catalan Oncology Institute (ICO), within the Trias i Pujol Hospital in Badalona (Barcelona). Very recently, he has taken part as a researcher for the ICO and the Josep Carreras Leukaemia Institute in a worldwide clinical trial (phase II) for the evaluation of a treatment with a new drug, Blinatumomab*, in acute Precursor B-cell lymphoblastic leukaemia patients in relapse. Thanks to the results of this study, recently published in Lancet Oncology, in which 189 ill adults from different international centres have taken part, the American Drug agency (FDA) approved its standard use a few weeks ago.
The patients who can be subject to this treatment are adults diagnosed with acute lymphoblastic leukaemia (LAL) either refractory (resistant to conventional treatment) or in relapse. The administration of Blinatumomab, a bispecific monoclonal antibody, has shown a complete response rate in 43% of the patients.
Currently a phase III study is taking place, which compares Blinatumomab to the best rescue chemotherapy these patients are receiving today, in order to achieve the definitive approval of Blinatumomab in refractory or relapsing LAL patients. The Josep Carreras Leukaemia Institute and the Catalan Oncology Institute in Badalona are also taking part in this trial.
Soon, Blinatumomab will be subjected to the approval of the European Medicines Agency (EMA).
* +info: Blinatumomab is a bispecific monoclonal antibody, anti-CD3 and anti-CD19, which is attached to the leukemic cell (CD19 positive) and to the T cytotoxic (CD3 positive) lymphocyte, so the T lymphocyte kills the leukemic cell directly. This proves the high efficiency of immunotherapy in acute lymphoblastic leukaemia, just like in other haematologic neoplasms.
Dr. Jordi Petriz, renowned international flow cytometry specialist, will join the Josep Carreras Leukaemia Research Institute.
Starting this January, the Josep Carreras Leukaemia Research Institute (IJC) will have a new high-profile research team focused on flow cytometry, which will add important experience and great recognition in the field, brought by biologist Dr. Jordi Petriz.
Dr.Petriz, who has been an expert in this discipline for more than two decades, has a broad international career, although his decision and commitment to continue developing his investigations in his home country are worth mentioning, for he has become one of the main representatives of flow cytometry in Spain.
This Catalan researcher who has developed his career as a researcher in the Vall d’Hebron Hospital Research Institute (VHIR) over the last few years, is internationally renown for his contribution to the field of study of stem cells, both for developing techniques to isolate and cultivate them and for the understanding of their biological process. He is also known for his contribution to the study of tumour development.
Recently, Dr. Petriz and his team’s interests focus on the basic mechanisms that regulate stem cells, examining the different genes involved in different aspects of their activation, and their resistance to multiple drugs. Their projects also use flow cytometry to analyse the genes that regulate cancer infiltration into the bone marrow. It is because of this that the Josep Carreras Leukaemia Institute was interested in this new team, in order to research flow cytometry further, a field that can offer new chances within the investigation of leukaemia and other malignant haemopaties.
Dr. Petriz’s team plays an essential role in encouraging the exchange between science and research and industry. The Josep Carreras Leukaemia Institute was established and is developed on the basis of translational research that strives to transfer the results of basic research to clinical implementation. Flow cytometry can be based on diagnosis, detecting interesting cell populations, counting them, marking them and/or isolating them depending on specific properties. Its uses are highly interesting in relation to designing new clinical trials with the pharmaceutical industry’s support in order to test new drugs that might improve the quality of life of the patients, maximise their response to the treatments and even cure the disease.
Because of this, Dr. Petriz will work very closely with all the other teams in the IJC, focused on the research of diseases such as leukaemia, lymphomas, multiple myeloma or myelodysplastic syndromes, among other haematologic neoplasms.
The common strategic objectives of the IJC are to understand the origins and development of leukaemia and other haematologic diseases, to identify new therapeutic goals for applying more precise and less aggressive treatments and to contribute these diseases becoming curable in 100% of the cases.
Crowdfunding the research of a bad prognosis type of childhood leukemia
From the Josep Carreras Leukemia Research Institute, Dr. Pablo Menéndez and his team are hard at work on studying a certain type of childhood leukemia with prenatal origin. Lymphoblastic pro-B leukemia in lactating children is a childhood tumor that has a very poor prognosis today.
This childhood leukemia is characterized by its high resistance to current drugs. Due to it being a cancer of little relevance, which affects few children a year in Spain, the resources allocated to researching the how and why of paediatric cancer are scarce.
MLL-AF4 lymphoblastic leukemia is diagnosed in children during their first year. This mutation is present at birth in 100% of the babies, which proves it appears during pregnancy. Even so, we do not know whether there are other alterations or genetic or epigenetic insults that take place soon after the birth and speed up the disease.
From the Josep Carreras Leukemia Research Institute, this line of investigation was recently opened in order to answer two questions key to improving the prognosis of babies who suffer from this very acute form of leukemia.
o Finding out in which blood cell this leukemia originates during prenatal development (during pregnancy)
o Finding out which cell is responsible for the infiltration into the central nervous system (the brain) babies with this form of leukemia usually suffer.
If we manage to identify the population phenotype responsible for the leukemic cells invading the central nervous system we will be able to identity the antigenic profile towards which we should direct new therapeutic strategies. This will undoubtedly allow us to improve the quality of life of the children suffering from this acute leukemia.
To be able to undertake this investigation we have decided to publish our project in the www.precipita.es website. Through this portal it is possible to make micro-donations so the project can be accomplished. With everyone’s help we will be able to reach the 25.000€ that will help us to continue fighting unrelentingly against leukemia.
CONTRIBUTE NOW
Precipita is a platform promoted by the Spanish Foundation of Science and Technology, FECYT, whose goal is to bring researchers and people interested in science together.
New Publication in Stem Cell Reports: Fast and Efficient Neural Conversion of Human Hematopoietic Cells
Dr. Alessandra Giorgetti, an associate researcher in the Stem Cells, Mesenchymal Cancer and Development group, led by Dr. Pablo Menéndez, has published a paper in the journal Stem Cell Reports this month.
The work describes a new way to generate neurons from blood stem cells (these cells give rise to all different types of cells in the blood). Neurons are specialist cells of the nervous system that transmit messages through electrical and chemical signals. The group is interested in generating populations of cells this way in order to use them to study the mechanisms of normal cell development, but also to see what goes wrong when cells turn leukaemic. Cells made this way, in the laboratory are also a safer and quicker way to test possible drugs and treatments in the first phases to check safety and see if they work.
In this paper the scientists describe a new method, which is much quicker and more efficient than those previously used to generate neurons in the laboratory. They use a sophisticated technique to introduce genes into the blood cells; these genes send signals that drive the conversion of blood cells into neurons. Most importantly these cells are capable of sending electrical signals in the same way that normal neurons do making them a good model that reproduces some of the characteristics of the nervous system in humans.
The cells will provide a valuable resource and a tool to test possible therapies for toxicity and efficacy of new drugs. These studies are essential before a possible treatment can be tried on patients, or even on mice.